Brief History of Thermoelectrics
Thermoelectric Effects - Early study of Thermoelectricity 1820-1920
In the 100 years before the world wars thermoelectricity was discovered and developed in western Europe by academic scientists, with much of the activity centered in Berlin.
Seebeck Effect
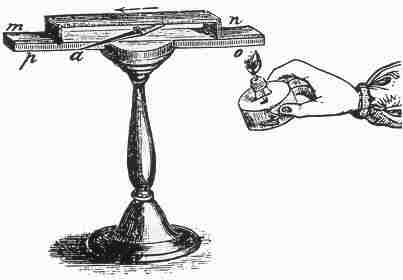
In 1821-3 Thomas Johann Seebeck found that a circuit made from two dissimilar metals, with junctions at different temperatures would deflect a compass magnet [1]. Seebeck initially believed this was due to magnetism induced by the temperature difference and thought it might be related to the Earth's magnetic field. However, it was quickly realized that a "Thermoelectric Force" induced an electrical current, which by Ampree's law deflects the magnet. More specifically, the temperature difference produces and electric potential (voltage) which can drive an electric current in a closed circuit. Today, this is known as the Seebeck effect.
|
|
Instrument used by Seebeck to observe the deflection of a compass needle (a) due to a thermoelectric induced current from heating the junction of two different metals (n and o). |
The voltage produced is proportional to the temperature difference between the two junctions. The proportionality constant (S or a) is known as the Seebeck coefficient, and often referred to as "thermopower" even though it is more related to potential than power. In 1851 Gustav Magnus discovered the Seebeck voltage does not depend on the distribution of temperature along the metals between the junctions [2] an indication that the thermopower is a thermodynamic state function. This is the physical basis for a thermocouple, which is used often for temperature measurement.
|
|
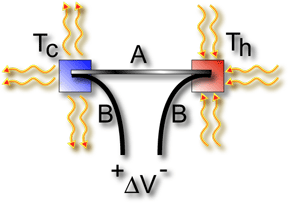 V = a(Th - Tc) The voltage
difference, V, produced across the terminals of an open circuit
made from a pair of dissimilar metals, A and B, whose two junctions
are held at different temperatures, is directly proportional to
the difference between the hot and cold junction temperatures, Th
- Tc [2]. |
Seebeck surveyed many different materials: elements, alloys and minerals including zinc antimonide, chalcogenide minerals such as PbS and cobalt arsenide and made a qualitative ordering of their relative Seebeck effect. In 1910 Werner Haken, following the studies on ZnSb and CdSb of Becquerel [3], quantitatively characterized the Seebeck coefficient (now in uV/K) and electrical conductivity of many elements, alloys and compounds correctly identifying Sb2Te3, Bi2Te3, Bi0.9Sb0.1, SnTe, Cu-Ni alloys as good thermoelectric materials and even studied PbTe [4].
|
Peltier Effect |
 Jean Charles A. Peltier |
|
Thomson Effect |
 William Thomson (Lord Kelvin) |
Edmund Altenkirch was the first to use the constant property model to derive the maximum efficiency of a thermoelectric generator (1909) as well as the performance of a cooler (1911) when the design and operating conditions are fully optimized [6]. This correct relationship, later developed into the 'figure of merit' zT, that good thermoelectric materials should possess large Seebeck coefficients, high electrical conductivity (to minimize Joule heating due to electrical resistance) and low thermal conductivity (to minimize heat loss). Early thermal conductivity measurements by A Eucken [7] on solids quickly revealed that point defects found in alloys significantly reduces lattice thermal conductivity - a strategy that becomes important for thermoelectric materials.
Thermoelectric Applications - Excitement and Disappointment 1920 - 1970
During and after the world wars thermoelectricity was actively studied for use in valuable technologies, primarily cooling as well as power generation for military as well as civilian uses. The political and economic importance of such devices made advances more difficult and slow to publicise particularly between the Eastern European and Western countries. By the 1950's, generator efficiencies had reached 5% and cooling from ambient to below 0 C was demonstrated which has ultimately lead to some viable industries. Many thought thermoelectrics would soon replace conventional heat engines and refrigeration and interest and research in thermoelectricity grew rapidly at major appliance corporations such as Westinghouse, universities and national research laboratories [8]. However, by the end of the 1960's the pace of progress had slowed with some discussion that the upper limit of zT might be near 1 and many research programs were dismantled (despite several reports of zT > 1).
|
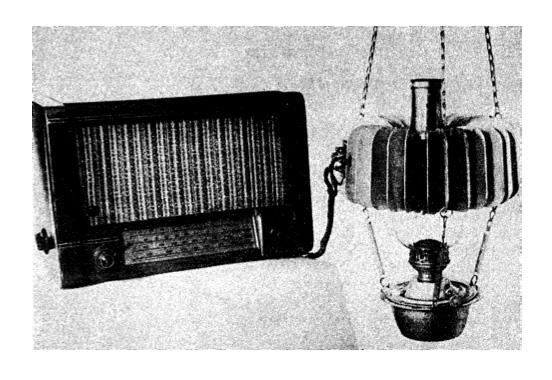 Oil burning lamp powering a radio using the first commercial thermoelectric generator containing ZnSb and constantan built in USSR beginning in 1948 [9] |
Figure of Merit zT
In 1949 Abram Fedorovich Ioffe developed the modern theory of thermoelectricity using the concept of the 'figure of merit' zT, culminating in the classic texts on Semiconductor Thermoelements and Thermoelectric Cooling (1956) [9].
Ioffe also promoted the use of the semiconductors in thermoelectrics and semiconductor physics to analyze results and optimize performance.
Materials with high thermoelectric figures of merit are typically heavily
doped semiconductors, the best known are the tellurides of antimony,
bismuth and lead. Ioffe and his institute in Saint Petersburg actively pursued thermoelectric research and development in USSR leading to some of the first commercial thermoelectric power generation and cooling devices. Ioffe was one of the first to promote the use of alloying to reduce lattice thermal conductivity by point defects.
| One of the first demonstrations of 0 C cooling was by H. Julian Goldsmid in 1954 using thermoelements based on Bi2Te3 [10]. Goldsmid was one of the first to utilize the thermoelectric quality factor, identifying the importance of high mobility and effective mass combination and low lattice thermal conductivity in semiconductors that when properly doped make good thermoelectric materials. Goldsmid authored many introductory books including Introduction to Thermoelecricity (2010). |
|
In the search for high zT materials, a general strategy guided by the quality factor has been to look for small band gap semiconductors made from heavy elements. Glen Slack summarized the material requirements succintly in the "phonon-glass electron-crystal" concept that the phonons should be disrupted like in a glass but the electrons should have high mobility like they do in crystalline semiconductors [11].
Thermoelectric Industry - Niche Applications 1970 - 2000
The reliability and simplicity of thermoelectricity enables niche applications for this solid-state technology even while conventional processes are more efficient. Besides thermocouples, a small but stable industry to produce Peltier coolers based on Bi2Te3-Sb2Te3 formed which now produce coolers for a variety of products ranging from optoelectronics, small refrigerators and seat cooling/heating systems. The need for reliable, remote power sources provids some niche applications for thermoelectric power generation.
The maturity of the science, technology and commercial use of thermoelectricity has lead to a number of focused scientific meetings and organizations, the largest of which is the International Thermoelectric Society with meetings since 1970.
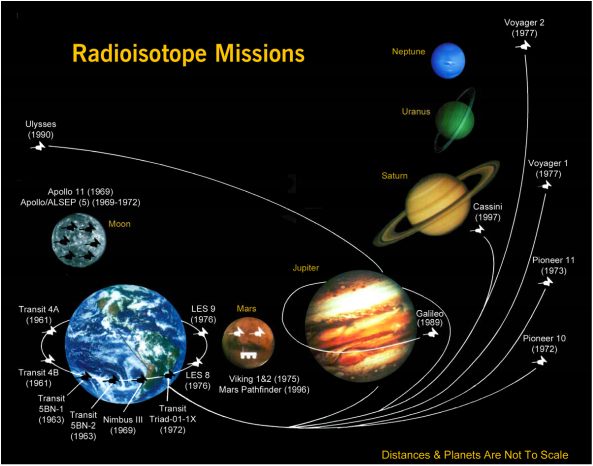
USA missions utilizing Radioisotope Thermoelectric Generators for Electrical Power
|
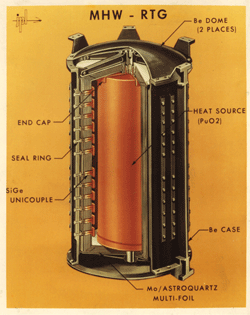
Thermoelectric Generators for Space For Space Exploration missions, particularly beyond the planet Mars, the light from the sun is too weak to power a spacecraft with solar panels. Instead, the electrical power is provided by converting the heat from a Pu238 heat source into electricity using thermoelectric couples. Such Radioisotope Thermoelectric Generators (RTG) have been used by NASA in a variety of missions such as Apollo, Pioneer, Viking, Voyager, Galileo and Cassini. With no moving parts, the power sources for Voyager are still operating, allowing the spacecraft to continue to make scientific discoveries after over 35 years of operation. The Curiosity rover on Mars is the first rover powered by thermoelectrics using a Multi-Mission RTG (MMRTG). |
|
New Concepts in Thermoelectricity 2000-
Interest in thermoelectricity renewed in the 1990's with the influx of new ideas. The hope that engineered structures will improve zT, particularly at the nanometer scale has reinvigorated research in thermoelectric materials [12]. While some of these ideas have shown to be ineffective, others have lead to entirely new classes of complex thermoelectric materials [13].
The global need for alternative sources of energy has revived interest in commercial applications [14] (see Energy Sustainability Page) and stimulated interest in developing inexpensive and environmentally-friendly thermoelectric materials.
REFERENCES
[1] Th. J. Seebeck "Magnetische
Polarisation der Metalle und Erze Durch Temperatur-Differenz"1822-23
in Ostwald's
Klassiker der Exakten Wissenshaften Nr. 70 (1895). Seebeck Biography 1. Seebeck Biography 2.
[2] G. Magnus, Poggendorf's Annalen der Physik 83 p469 (1851)
[3] E. Becquerel, Ann. de chim. et phys. (4) 8. (1866)
[4] W. Haken, Annalen der Physik 832 p291-336 (1910)
[5] W. Thomson "On the Dynamical Theory of Heat. Trans." R. Soc. Edinburgh: Earth Sci. 3,
91–98 (1851). Thomson Biography.
[6] E. Altenkirch, Physikalische Zeitschrift 10, 560–580 (1909); Physikalische Zeitschrift 12, 920 (1911)
[7] A. Eucken and G. Kuhn Z. Phys. Chem. 134 p193 (1928)
[8] R. R. Heikes and R. W. Ur, "Thermoelectricity: Science and Engineering" Interscience
Publishers, (1961); I. B. Cadoff and E. Miller "Thermoelectric Materials and Devices"
Materials Technology Series. Reinhold Publishing Cooperation (1960); P. H. Egli "Thermoelectricity" John Wiley & Sons (1960)
[9] M. V. Vedernikov and E. K. Iordanishvili "A. F. Ioffe and origin of modern semiconductor thermoelectric energy conversion" 17th Int. Conf. on Thermoelectrics vol 1, pp 37–42 (1998); A. F. Ioffe "Semiconductor Thermoelements and Thermoelectric Cooling"
[10] H. J. Goldsmid and R. W. Douglas "The use of semiconductors in thermoelectric refrigeration" British J. Appl. Phys. 5, 386 (1954)
[11] Glen Slack in CRC Handbook of Thermoelectrics (ed. Rowe, M.) p407–440 (1995).
[12] M.S. Dresselhaus, et al. "New directions for low-dimensional thermoelectric
materials" Adv. Mater. 19, 1043–1053 (2007); M. G. Kanatzidis "Nanostructured thermoelectrics: the new paradigm?" Chem. Mater. 22, 648–659 (2010)
[13] G. J. Snyder and E. Toberer "Complex Thermoelectric Materials" Nature Materials 7, 105-114 (2008)
[14] L. Bell "Cooling, Heating, Generating Power, and Recovering Waste Heat with Thermoelectric Systems" Science Vol. 321. pp. 1457 (2008)